| navigation: | Home | Copyright | Contact us | | |
|
|
Enzymes:
1. Hexokinase
2. Phosphoglucoisomerase
3. Phosphofructokinase
4. Aldolase
5. Triose phosphate isomerase
6. Glyceraldehyde-3-phosphate dehydrogenase
7. Phosphoglycerate kinase
8. Phosphoglycerate mutase
9. Enolase
10. Pyruvate kinase
Compounds:
ATP
ADP
Glucose
Pyruvate
Glucose
published: 21 Nov 2011 (16:36)
Glucose an overview
Glucose belongs to one of the greatest class of chemical compounds, the carbohydrates or saccharides, or to be more precise, the monosaccharides. The name carbohydrates represents the fact that many saccharides can be shown by the simple stoichiometric formula (CH2O)n.Glucose plays an important role as a structural element of many biological materials. Here are some important examples of biological substances that are glucose or other saccharides in whole or in part: cellulose of woody plants, exoskeletons of insects and other arthropods and cell walls of bacteria are the most important examples. Amylose, amylopectin and glycogen are stored polysaccharides in plants and animals, respectively. These compounds are homopolysaccharides of the class called glucans, the polymers of glucose. Another important role of polysaccharides is the formation of glycoproteins - proteins with covalently linked oligosaccharides.
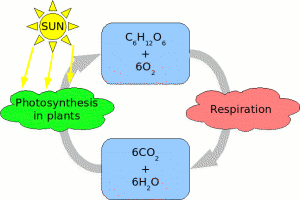 |
| Glucose cycle in biosphere. |
Carbohydrates and especially glucose play a central role in the biosphere energetic cycle as a first organic molecule synthesised by plants during photosynthesis from energy of the light and carbon dioxide CO2. Animals and bacterial cells obtain carbohydrates through digestion of plants. Carbon dioxide or CO2 and water from respiration cycle are the final products or the glucose utilization by animals. Animals can digest glucose in a form of mono- and polysaccharides.
Glucose general data
| Chemical name: | 6-(hydroxymethyl)oxane-2,3,4,5-tetrol |
| Molecular mass: | 180.16 g/mol |
| Density: | 1.54 gxcm-3 |
| Melting point: | α-D-Glucose: 146°C β-D-Glucose: 150°C |
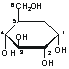
Glucose chemical structure
Glucose brutal formula
Glucose is a monosaccharides with brutal formula (CH2O)6 or in more convenient format: C6H12O6 contains sic carbon atoms and one aldehyde group and therefore classified as an aldohexose.Glucose mutarotation
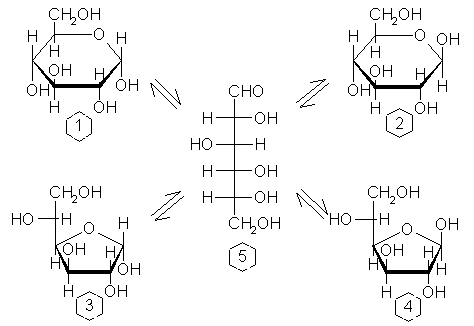 |
| Diagram of Glucose mutoratation in solution. Glucose tautmers.1. α-D-Glucopyranose (62%); 2. β-D-Glucopyranose (38%); 3. α-D-Glucofuranose (trace); 4. β-D-Glucofuranose (trace); 5. Linear D-Glucose (0.01%). |
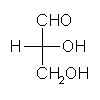
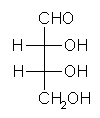
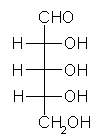
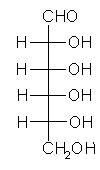
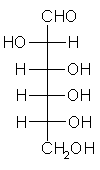
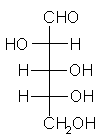
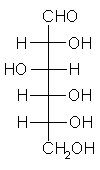
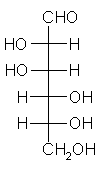
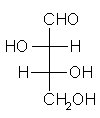
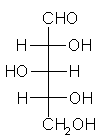
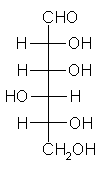
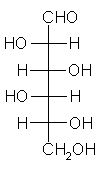
General relations within D-aldose family
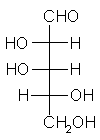
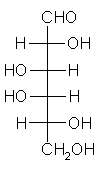
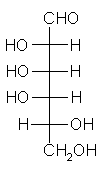
| Triose carbohydrate C3H6O3 Mr: 90.08 | Tetrose carbohydrate C4H8O4 Mr: 120.10 | Pentose carbohydrate (Aldopentoses) C5H10O5 Mr: 150.13 | Hexose carbohydrate (Aldohexoses) C6H12O6 Mr: 180.16 | ||||||||
|
|
|
| ||||||||
| |||||||||||
|
| ||||||||||
| |||||||||||
|
|
| |||||||||
| |||||||||||
|
| ||||||||||
|
News
Inhibit Glycolysis to Starve Tumour CellsThe multi-system disease called Tuberous ...
Glycolysis Role in RhabdomyosarcomasA study conducted by the IDIBELL researchers ...
Other news pages:
1
| Copyright 2007-... by Glycolysis.co.uk |